Abstract
Introduction: Pediatric Severe Aplastic Anemia (sAA) results in severe and prolonged pancytopenia that may increase the risk for infections. Infections from bacterial, viral, and fungal etiologies remain a leading cause of mortality, prompting an interest in the development of infectious disease (ID) supportive care guidelines. However, institutional clinical practice guidelines (CPG) to-date are often extrapolated from adult studies or providers experiences with other oncologic diagnoses given the paucity of sAA-specific data to inform best practices.
Objective: Evaluate current antimicrobial prophylaxis practices in individuals with sAA or very severe aplastic anemia (vsAA) receiving care at hematology centers participating in the North American Pediatric Aplastic Anemia Consortium (NAPAAC).
Method: A RedCap survey was created and electronically distributed to the 53 NAPAAC institutions querying ID antimicrobial prophylaxis practices in pediatric patients with sAA /vsAA. Pediatric patients were defined as individuals 0 to 18 years of age who had not received a hematopoietic cell transplant (HCT) and met diagnostic criteria for sAA/ vsAA. Standard immunosuppressive therapy (IST) agents included anti-thymocyte globulin, calcineurin inhibitors, alemtuzumab, eltromobag (not monotherapy), and systemic corticosteroids. Each institution was asked to submit one collective survey response from June 1, 2022 to July 16, 2022.
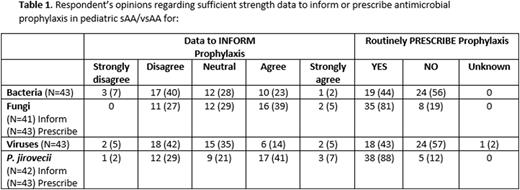
Results: In total 43 (81%) NAPAAC sites completed the survey. The participant demographics were as follows: 68% (N=29) hematologists, 30% (N=13) HCT specialists, 2% (N=1) practiced as both. Most centers diagnosed 0 to 5 new sAA/vsAA cases/ year (N=28, 65%), 3 (7%) centers diagnosed ≥11/yr. Centers reported having a written CPG for bacterial (N=11; 26%), viral (N=11; 26%), fungal (N=19; 44%), and Pneumocystis (PCP) prophylaxis (N=17; 41%). Respondent opinions regarding strength of data to inform and prescribe ID prophylaxis are summarized in Table 1. Less than half of respondents felt there was sufficient data to inform the decision to prescribe antimicrobial prophylaxis. In the 44% of centers that prescribe antibacterial prophylaxis, absolute neutrophil count (ANC) threshold of 500 was the most frequent criterion used to start and stop prophylaxis. The preferred antibacterial prophylaxis agent was levofloxacin (N=29, 91%) among centers regardless of beta-lactam allergy and the majority of centers did not use IVIG (N=37, 86%). Thirty-four (79%) centers perform antiviral testing at diagnosis and 43% reported prescribing antiviral prophylaxis, preferentially initiating acyclovir in HSV or VZV seropositive patients who receive IST. Twelve (28%) centers routinely performed viral PCR monitoring (frequency of twice weekly to monthly) for CMV. Eighty-one percent of institutions prescribe fungal prophylaxis, most frequently with mold active agents (N=24, 62%) over fluconazole (N=12, 31%). Among mold-active agents, voriconazole was preferentially prescribed (N=19, 79%); dosing was below the recommended maintenance amount of 8-9 mg/kg/dose in 65% of prescribing centers. Criteria to discontinue antifungal prophylaxis included a combination of time after IST completion (0-3 months) and ANC ≥ 500 (N=22, 51%) or ALC > 1000 (N=5, 12%). Eighty-eight percent of centers prescribe PCP prophylaxis with IV pentamidine (N=12, 32%), aerosolized pentamidine (N=10, 26%), or TMP/S (N=11, 29%) based primarily on initiation IST, less on ALC. Cessation of PCP prophylaxis was variable with 60% either stopping 3 or 6 months after completion of IST; ANC or ALC contributed less frequently to the decision to stop PCP prophylaxis.
Conclusion: This study evaluated current antimicrobial prophylaxis practices from multiple NAPAAC institutions, highlighting the degree of practice variation across centers caring for pediatric patients with sAA or vsAA. Interestingly, the majority of centers do not have written ID prophylaxis guidelines in place and in centers with a prophylaxis CPG for this population, practice variability persists regarding type of prophylaxis, preferred agents, and criteria to start and stop prophylaxis. This study underscores the need for sAA/vsAA-specific data to inform national guidelines regarding optimal antimicrobial prophylaxis strategies in these high-risk children.
Disclosures
Rothman:bluebird bio: Research Funding; Novartis: Honoraria, Research Funding; Agios: Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal